Targeting the Cytotoxicity of Topoisomerase 11-directed Epipodophyllotoxins to Tumor Cells in Acidic Environments
Peter Buhi Jensen, Boe Sandahi Sorensen,Maxwell Sehested, Pernille Grue, Erland J. F. Demant, and Heine H. Hansen
Department of Oncology, The Finsen Institute Rigshospitale, 9 Blegdamsvej, DK-2100 Copenhagen [P. B. J., P. G., H. H. H.]; Department of Molecular Biology, University of Aarhus, DK-800 Aarhus [B. S. S.]; Department of Pathology, Sundby Hospital, DK-2300 Copenhagen [M. S.]; and Department of Medical Biochemistry and Genetics, University of Copenhagen DK-2100 Copenhagen [E. J. F. D.], Denmark
Solid tumors differ from normal tissue in that the extracellular pH (pHe) of a solid tumor is typically at least 0.5 lower than that of normal tissue (pHe = 7.4). By combining the drugs etoposide (VP-16) and chloroquine (CQ), selective delivery of toxins to tumor cells can be achieved.
Etoposide is a commonly used chemotherapy agent that interferes with the actions of topoisomerase II. Topoisomerase II is an essential class of nuclear proteins that unwinds supercoiled DNA by briefly cutting both strands of the DNA double-helix and rejoins them after relaxing the tension in the DNA. Etoposide stabilizes an intermediate state in this process, leaving both strands of the DNA cut and prevents topoisomerase II from rejoining the strands. Etoposide’s mechanism of action is not pH-sensitive and, when used alone in treatment, affects both normal and cancerous cells equally.


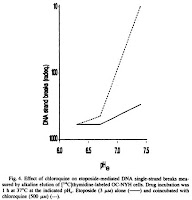
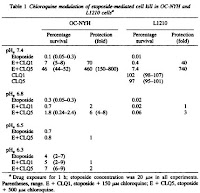
Because of its sensitivity to pH, chloroquine is largely sequestered extracellularly in the acidic extracellular tumor environments relative to neutral extracellular environments observed in most other body tissues. As a result, chloroquine tends to intercalate DNA within normal cells in the body, protecting them from etoposide. However there is little or no protective effect for tumor cells because chloroquine is unable to enter cancerous cells due to an acidic extracellular pH. This study confirms this hypothesis by varying extracellular pH in cell cultures treated with etoposide and choroquine (Fig.3) (Fig.4). Comparisons of pHe = 7.4 and pHe = 6.5 revealed a 460-fold protection (Table 1).

Significance & Future Work:
Topoisomerase II is especially prevalent in cancerous cells and is a vital mechanism for cellular proliferation and gene expression. The etoposide-chloroquine drug combination allows selective killing of cancerous cells in a systemic manner while minimizing harm to normal cells within the body. This combination is advantageous to simply etoposide because the protective effect of chloroquine allows dose increments of etoposide to increase effectiveness of the treatment and better combat drug-resistant cancers. Other selective methods have attempted to target tumor-specific characteristics such as membrane receptors, but cancerous cells have only a small number of tumor-specific alterations. Thus, this is not only a more feasible treatment option, but it is also lower-cost. Unfortunately, chloroquine itself is toxic at certain doses. Additional studies have been performed to identify chloroquine derivatives that maintain pH-sensitivity but have lower levels of toxicity.

11 comments:
How exactly is chloroquine toxic to the normal cells? What types of tumors are not "solid" such that this treatment is not feasible? What is the pKa of chloroquine?
I believe chloroquine may cause damage to cell membranes in such a way that eventually leads to apoptosis.
I'm a bit curious about the clinical applications of using chloroquine as an "enhancer" drug. Andrew pointed out something interesting in that not all cancers result in solid tumors, such as (I'm guessing) leukemia. But for solid tumors, this seems quite promising.
I do see one issue though. I'm guessing here, but are tumor ECM's more acidic because the area isn't perfused well enough with oxygen? Less oxygen -> more CO2 -> more acidic? If that's the case, then once these tumors shrink to a certain size, then the pH will return to neutral and then this drug combination will lose potency? But I could still see this as being useful, since some tumor-removal operations can only be done if tumors are below a certain size, and I could see chemotherapy being used to shrink tumors so that they're removable through surgery.
Do the authors state a maximum dosage of the etoposide-chloroquine drug combination such that chloroquine does not reach a toxic level in the body? With the danger of injecting too much chloroquine, it seems that the localization of drug through some sort of tumor-targeting delivery method may allow for higher dosages of the anti-tumor drug, increasing the effectiveness and potency of the treatment. For example, tumors express a high concentration of folate receptors on their surface and by using folic acid and a polymeric drug delivery device, more anti-tumor drug can be injected without worrying about the side-effects of another drug.
Also, are there any signs of chloroquine becoming inhibited by lysosomes, which have a low pH internal compartment?
Looking at chloroquine studies based on the drug's anti-malarial activity, safe dosages of chloroquine (~300 mg to 600 mg) result in blood concentrations of around 500 nM [1]. Unless the concentration of chloroquine in tissue is much higher than in blood (which may be the case - I'm not sure), it appears that concentrations used in this study are not clinically relevant. Figure 1 shows the effects of chloroquine as a chemotherapy 'enhancer' on human lung cancer cells - the concentrations used are on the order of 100s of uM and the protecting effects of chloroquine (in the presence of chemotherapy agent etoposide) begins to fade below 600 uM. These concentrations seem too high for practical clinical application.
Does the paper mention anything about safe chloroquine concentrations in the tissue or whether the concentrations they used are clinically feasible?
[1] http://www.ncbi.nlm.nih.gov/pmc/articles/PMC89779/
Personally, I would have preferred more details about the methods in the summary.
To build on what Harry was saying above, it seems reasonable to say that the treatment loses effectiveness as the tumor shrinks. If this is true, it better be hoped that other methods finish it off, because otherwise you're fostering easy drug resistance, and it probably can't be used more than once or twice.
In response to many of the comments and with a point of my own, from the paper it seems that chloroquine interacts with DNA and jams up etoposides such that the etoposides cannot function. In this sense is it fair to say that the a lot of the chloroquine is sequestered by the etoposide?
Furthermore, if a significant amount of chloroquine is sequestered by the etoposide, is there any evidence that the safe concentration of chloroquine with etoposide is higher than normal?
Lastly, I believe that chloroquine is used in many chemotherapy cocktails which leads me to believe it is feasible "enhancer." I wonder what information in the clinical trials lead people to believe that chloroquine is a worthy "enhancer."
The paper talks about how a given cell is only destroyed if it is targeted by both Chloroquine and Etoposide. However, does the paper mention how these two drugs are administered? For example, are both administered simultaneously intraveneously? How does one ensure the drugs are absorbed equally by all organs in the body/cancer cells? Are the mechanisms by which the two drugs act on the cells similar enough for one to assume cancer cells are equally exposed/susceptible to both drugs?
It would be interesting to explore the idea of Chloroquine and Etoposide working together, but achieve another level of specific targeting by delivering the drugs to the tumor site by various drug delivery mechanisms such as viral vector or nanoparticle delivery devices. Another alternative is to look at delivering a prodrug form of Chloroquine that could be activated near tumor sites treated with Etoposide so as to reduce the toxic effects of Chloroquine on healthy cells.
Andrew, chloroquine is a weak base with a pka=8.5. I didn’t mention it in my analysis, but chloroquine has been shown to enhance the effects of imatinab (Gleevec) which is a miracle drug for chronic myeloid leukemia (CML) patients. Because chloroquine also acts as an autophagy inhibitor, it prevents a cell from recycling its organelles if too much chloroquine accumulates in the lysosome. This will then make it more difficult for cells treated with toxins to escape cell death. This is especially interesting because it acts as an enhancer in this chemotherapy combo rather than as a protectant as with the case of chloroquine with etoposide.
Harry, you are on the right track with your reasoning of the pH. Because cancer cells have such high rates of metabolism, they produce a good deal of waste (i.e. CO2), as a result, the immediate environment of these cells will have a slightly lower pH. I have not read of this drug combination losing potency, but we must remember that both of these drugs can have very detrimental effects upon the human body if they are given for prolongued periods of time, so we must be careful in its usage.
Brian, the authors don’t state a maximum dosage for the body as these tests were performed on mouse leukemia cell lines. I agree that too much chloroquine is a bad thing, especially since chloroquine has been shown to induce cell death just by itself. It may be possible that there are other drug-delivery methods that can be used as cancer treatment, but the more methods we have, the more aggressive chemotherapy regimens can be prepared by physicians. Each and every new therapy provides a new tool that may be used in combination with existing tools to combat cancer.
For those of you who are hungry for more detail on methods: The study was performed on L1210 mouse leukemia cells and OC-NYH human small cell lung cancer cell line. Chloroquine was used at 30 mg/mL, which I find to be a very high concentration, and Etoposide was administered at 20 mg/mL. Cell viability was determined by looking at dye exclusion in the hemocytometer.
John, if you look at the last figure (Table 1), the difference between conditions at pH=6.5 and pH=7.4 show a 460-fold protection effect. This indicates that a much higher concentration of etoposide can be safely used when chloroquine is also administered. As for safe levels of chloroquine, it would likely depend on the application. Chloroquine has been shown to diffuse and accumulate
Vaibhavi, I think your idea of delivering a prodrug form is an excellent thought. However, one must be careful in designing such a prodrug as the specific quinoline portions of the drug must not be inhibited from its action in any way lest it’s properties in being sequestered in acidic environments or its ability to diffuse across cell membranes be altered.
That's a pretty interesting drug delivery technique.
I just looked up the paper and saw that it was published in 1994. Have they had any more developments on this technique as an anticancer therapy? Just curious to see if they expanded their research on this topic or if it didn't work out. If they have developed this technique, have they expanded it to in vivo animal models?
Post a Comment