The oncogenic roles of Notch1 in astrocytic gliomas in vitro and in vivo
The oncogenic roles of Notch1 in astrocytic gliomas
in vitro and in vivo
Peng Xu Æ Mingzhe Qiu Æ Zhiyong Zhang Æ
Chunsheng Kang Æ Rongcai Jiang Æ Zhifan Jia Æ
Guangxiu Wang Æ Hao Jiang Æ Peiyu Pu
J Neurooncol. September 8, 2009
© Springer Science+Business Media, LLC. 2009
Summary
The Notch signaling pathway plays an important role in cell proliferation and the determination of cell fate and apoptosis. This pathway contains four different Notch receptors (Notch 1-4) that are sensitive and responsive to different cell types. The Notch1 receptor contributes much of the Notch activated signaling in astrocytic gliomas (cancerous cells of the nervous system). Therefore, Notch activation is high in astrocytic gliomas via the Notch1 receptor being activated by cell surface Notch ligands (Delta and Jagged). This high level of Notch activation causes much cell proliferation in astroglial cells, ultimately causing astrocytic gliomas. Hence, the authors assess the oncogenic role of Notch1 activation in astrocytic gliomas by downregulating the Notch1 gene via siRNA in vitro and in vivo. Most importantly, they seek to investigate that the downregulation of Notch1 reduces the proliferation rate of the astrocytic gliomas. 
Figure 1.
Figure 1 illustrates the significant levels of Notch1, PCNA (proliferating cell nuclear antigen), and cyclin D1 in different grades of gliomas. PCNA and cyclin D1 are two important proteins that assist and indicate cell proliferation. Figure 1a shows mRNA Notch1 expression via RT-PCR. Markers 1-6 indicate WHO IV gliomas, 7-12 WHO III gliomas, 13-18 WHO II gliomas, and 19-22 normal brain tissues. In Fig. 1a it is important to note that Notch1 expression is very faint in normal brain tissues (19-22). Figure 1b shows the expression of the Notch1 protein in the different grades of gliomas plus normal brain tissues. It seems clearer in Figure 1b that Notch1 expression is higher in a higher stage glioma. Figure 1c shows the immunohistochemical expression (hematoxylin and eosin staining) of Notch1, cyclin D1, and PCNA of the three different grades of gliomas (WHO II, WHO III, WHO IV). It seems like Notch1, cyclin D1, and PCNA are all upregulated the most at the WHO IV stage glioma. However, Fig. 1c should have shown immunohistochemical staining of normal brain tissue to keep consistent with figures 1a and 1b. Nevertheless, Figure 1 indicates that gliomas and more severe gliomas contain higher levels of the expression of Notch1. 
Figure 2.
The assessment of the Notch1 knockdown expression in vitro is illustrated in Figure 2. Through the use of Notch1 siRNA on the U251-MG cell line (Human glioblastoma cells) it is illustrated in the immunoflourescent staining of Fig. 2a that Notch1 expression decreases in the glioblastoma cell line upon siRNA treatment. The same result is seen in a western blot in Fig 2b. Most importantly, figure 2 illustrates that a cross-talk exists between Notch and the EGFR signaling pathways. Upon downregulation of Notch activation this also downregulates EGFR activation and the components of its signaling cascade that are also shown in Figure 2. The control in Figure 2 is no siRNA and scr-si RNA (scrambled siRNA) acts as a negative control. 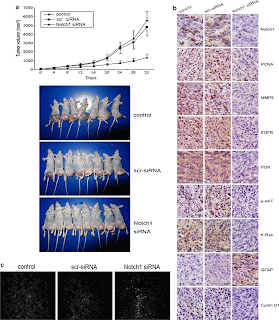
Figure 4.
Figure 4 illustrates the in-vivo study by utilizing a U251 subcutaneous glioma xenograft model treated with Notch1 siRNA. Figure 4a shows that the tumor volume of the Notch1 siRNA treated xenografts were much smaller at day 32 after implantation than in the controls. Immunohistochemical analysis again illustrates that the Notch1 siRNA treated tumors were downregulated in Notch1 and EGFR components compared to that of the controls. The only upregulated component in Fig. 4b is GFAP. GFAP is not part of the Notch or EGFR signaling pathways. However, GFAP (glial fibrillary acidic protein) is increased upon Notch1 siRNA, which illustrates more differentiation to normal glial cells. Figure 4c demonstrates the extent of apoptosis of the tumor cells through the labeling and indication of Annexin-V under a fluorescent microscope. Thus, Fig. 4c indicates that the tumors with Notch1 siRNA contain a higher number of apoptotic cells than the controls. In all, Figure 4 demonstrates that the knockdown of Notch1 in vivo serves to combat astrocytic gliomas.
Overall, this paper explains that high Notch activation via the Notch1 receptor in astrocytes causes these cells to proliferate excessively and become cancerous. Thus, the knockdown of Notch1 expression provides some hope to combat astrocytic gliomas. Furthermore, the authors illustrate that the knockdown of Notch1 expression by siRNA simultaneously downregulates the expression of EGFR (Epidermal Growth Factor Receptor) and the important components of its downstream pathway. This ultimately signifies an important cross-talk between Notch and EGFR signaling pathways. The activation of EGFR also promotes cell proliferation, but since EGFR is also downregulated when Notch1 is downregulated then this possibly shows a positive feedback loop in trying to halt the proliferation rate of the astrocytic gliomas.
Critique:
This paper introduces the concept of a Notch and EGFR signaling crosstalk, but does not explain much of the significance for the cross-talk. I would have liked to see what would happen in the tumors if EGFR was downregulated via a designed siRNA for EGFR. It is shown that if Notch1 is downregulated, then EGFR will also be downregulated. However, this does not explain if Notch directly affects EGFR. If EGFR is downregulated will Notch also be downregulated as well? Furthermore, in Figure 4 the authors do not state what stage type of glioma was implanted in the nude mice. In Figure 1 the authors make a claim that a higher stage glioma has higher Notch1 activity. The stages of the gliomas could affect the certain results shown in the immunohistochemical and annexin-V apoptosis analysis. Moreover, the authors only used 6 week old female immune-deficient nude mice for the in-vivo study. Cells react differently in different aged environments and it would have been informative to see how the downregulation of Notch1 in young mice compares to that of old mice. Lastly, the authors should have analyzed downstream genes of Notch (Hes and Hey) to confirm if Notch activation has been downregulated. The Hes and Hey genes are the target genes of Notch activation, and it would have been important to see which ones get upregulated or downregulated upon knocking down Notch1 expression in murine animals and mammals in general.
Significance:
This paper provides a promising direction on how to combat tumors through the manipulation of the Notch signaling pathway, which is responsible in determining cell proliferation and cell fate. Nevertheless, Notch signaling affects different types of tissues depending on which Notch receptor gets activated. In this case, the Notch1 receptor affects astrocytes and once it is highly activated it can cause brain tumors. However, in some instances a high activation of Notch can act as a tumor suppressor. Nonetheless, Notch signaling has an important role on cell proliferation rate and ultimately in the formations of certain tumors. Moreover, Notch may also be manipulated under other pathways that it could cross-talk with. Therefore, when trying to knockdown Notch activation another pathway could be knocked down that Notch could cross talk with. Specifically, this paper should look towards future experiments in figuring out the true mechanisms in the cross-talk between the Notch and EGFR signaling pathways, and how these pathways could affect other signaling pathways in tumor suppression. Also, since it is mentioned in the paper that all Notch signaling gets processed via an enzyme called gamma secretase, a gamma secretase inhibitor should be utilized in tumors to knockdown all of the Notch activation potential in the Notch receptors of Notch 1-4. This study may provide hopeful ways to combat any tumor.

8 comments:
If they correlate Notch1 activation to EGFr amount, do they ever look at EGFr activation levels (phosphorylated EGFr)?
They do look at EGFr levels, but not phosphorylated EGFr levels. They associate EGFr activation with phosphorylated molecules downstream of the EGF pathway such as the kinases of p-AKT and P13-K. However, it would have been nice to see directed levels of activated EGFr to also confirm that Notch has an important crosstalking mechanism with the EGFr signaling pathway.
Since the Notch1 pathway is only malignant when over-expressed in astroglia, would making a drug that silences the Notch1 have deleterious affects on neural growth in other parts of the body, especially when taking into account the cross-talking phenomena?
Yes. When utilizing gamma secretase inhibitor as a therapeutic drug to downregulate Notch activation, this could also be harmful to other cells if the drug was administered to a non targeted area of interest. Too much Notch signaling is bad and too little Notch signaling is bad. Therefore, there is an attenuation signal of Notch that needs to be maintained in all cells of the body. This must be assessed when trying to downregulate Notch in cancer cells.
While initial results in the paper include all 4 grades of gliomas, the later results dealing with the siRNA only use one control. What specifically is this contol - a grade IV glioma tumor or something else?
Also, the authors should have also included Cyclin D2 in their analysis. Cdkn2 has been shown to be down regulated or deleted in glioblastoma cells as compared to normal cells. Therefore, it would have been interesting to see how downregulating Notch 1 would affect cdkn2.
Furthermore, it should be noted that glioblastoma affects a greater number of males than femals - why then did the authors use female rats?
Do they ever discuss about a practical in vivo approach to safely deliver gamma secretase inhibitor to the astrocytic gliomas? If GSI is supposed to be used as a therapeutic agent, it would be important to assess the localized effects on the cancers.
The control was a certain human glioblastoma cell line. Other cdks would have been very interesting to check, but they only seemed to check cyclin D1. I did not know that glioblastoma affects more males than females. That is a very interesting point. The author does not state why they used female mice. Could you please send me more info about why male mice are more prone to glioblastomas?
No. They don't mention about a practical safe in vivo technique to deliver GSI in a localized area. However, Johntus that is a great point that should be assessed in future studies!
Post a Comment