In vitro and in vivo evaluation of osteogenesis of human umbilical cord blood derived mesenchymal stem cells on partially demineraliz
In vitro and in vivo evaluation of osteogenesis of human umbilical cord blood derived mesenchymal stem cells on partially demineralized bone matrix
GUANGPENG LIU, M.D., Ph.D., YULIN LI, B.S., JIAN SUN, B.S., HENG ZHOU, M.S., WENJIE ZHANG, M.D., Ph.D., LEI CUI, M.D., Ph.D., and YILIN CAO, M.D., Ph.D.
Summary:
When regeneration of bone defects is needed, bone grafts taken from the patient are usually used. However, when the bone defect is rather large, grafts cannot be done because not enough material can be extracted for the grant. One solution to this problem is using tissue engineering to develop a degradable scaffold that contains osteogenically differentiated mesenchymal stem cells to regrow the bone. These scaffolds are called partially demineralized bone matrix (pDBM). The purpose of this lab was to determine whether such a scaffold can support the osteogenic differentiation of mesenchymal stem cells obtained from umbilical cord blood (UCB-MSCs), which are easier to obtain than those from bone marrow (BMSC), both in vitro and in vivo.
First, human umbilical cord blood samples were taken and processed in heparnized tubes to avoid coagulation. The blood sample was diluted, and mononuclear cells were isolated using density gradient centrifugation. These cells were then seeded in dishes and grown in basic culture media. After the culture was fairly confluent, single separated colonies of fibroblast like cells were detached and replated (essentially picking out the cells most thought to be MSCs. The cells were then passaged up to 10 times for use. Cell quantification was done through the use of a DNA assay to determine the doubling time, which was about 33 hours for the UCB-MSCs. Further analysis of the MSC’s seeded were done using flow cytometry with various cell surface antigen markers to determine when the MSC cultures were most pure. MSC s were determined to be the populations that determined a homogenous, spindle-like population, and tested positive for the CD29, CD105, CD106, CD 166, and Stro-1 antigen markers.
The differentiation potential of the UCB-MSCs was determined by inducing osteogenic, adipogenic, and chrondrogeinc differentiation through various methods. Staining was used to determine whether differentiation occurred or not, and it was found that the UCB-MSCs readily differentiated in all 3 ways when induced, but did not differentiate spontaneously when not induced.
Meanwhile, the pDBM scaffolds were prepared from porcine trabecular bone. The bone samples were extracted in absolute ethanol, and then decullarized in TritonX-100, and demineralized in an HCl solution. The scaffolds were then dried and sterilized so that they could be seeded. Then, UCB-MSC samples were added onto the scaffold and incubated for 2 hours, after which the whole cell/scaffold composite was transferred to a separate well Some of these composites served as a control, with no induced differentiation, while others were cultured in osteogenic medium to induce osteogenesis. Sputter coating and SEM were used to visualize the culture growth on the scaffold, and DNA assays were used to determine the cell number of the culture. It was found that both induced and non induced cells spread and attached well to the pdBM scaffold, but the osteogenically induced cells grew for longer and to a higher total cell number than the non-induced ones.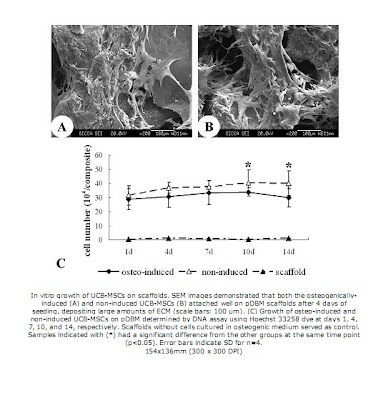 To assess the osteogenesis of the cells on the scaffolds, multiple tests were then done. First, tests for alkaline phosphatase (via phosphate substrate solution and absorbance readings) and osteocalcin (via ELISA) were performed, and it was found that the induced group of cells had increased alkaline phosphatase activity and osteocalcin levels when compared to the control. After 14 days of culture, RT-PCR was performed, and four genes related to osteoblasts, alkaline phosphatase, osteocalcin, osteopontin, and collagen type I were amplified. The induced cells were shown to be expressing all 4 of these genes, while the control cells did not express osteocalicn or osteopontin transcripts, and expressed the alkaline phosphatase at a severely reduced level.
To assess the osteogenesis of the cells on the scaffolds, multiple tests were then done. First, tests for alkaline phosphatase (via phosphate substrate solution and absorbance readings) and osteocalcin (via ELISA) were performed, and it was found that the induced group of cells had increased alkaline phosphatase activity and osteocalcin levels when compared to the control. After 14 days of culture, RT-PCR was performed, and four genes related to osteoblasts, alkaline phosphatase, osteocalcin, osteopontin, and collagen type I were amplified. The induced cells were shown to be expressing all 4 of these genes, while the control cells did not express osteocalicn or osteopontin transcripts, and expressed the alkaline phosphatase at a severely reduced level.
After this analysis, the in vitro part of the experiment was begun. A critical-sized bone defect was created in the rat’s skull, and the defect was then filled with an induced UCB-MSC/pDBM composite, with a non-induced composite, with just a pDBM scaffold, or nothing at all. Micro-CT scans, BMD readings, and histological tests were done of the inserted area 6 and 12 weeks after surgery. After 12 weeks, it was found that the defects filled with the induced complexes had started to form new bone tissue, and the pDBM scaffold was completely reabsorbed. Meanwhile, the other groups did not display much evidence of bone growth or union, and the scaffold (if present) was still present in the defect.
The BMD and histological results confirmed what the micro-CT scans showed. The bone formed from the induced complexes had a very high BMD, comparable to that of normal bone. The other two groups that had a scaffold inserted (but did not contain induced cells) had very low BMDs that actually decreased through the 12 weeks, most likely due to scaffold degeneration. When examined histologically, these results were further confirmed. After 6 weeks, the induced complex group formed new osteoid-like tissue in between the fragments of scaffold still left, while the other groups formed only thick fibrous tissue if a scaffold was present, or very thin fibrous tissue if no scaffold was present. After 12 weeks, the induced group displayed no residual scaffold, and the defect was almost completely bridged by bone, while the other groups showed only minimal bone growth.
Discussion and Critique:
In general, the differentiation of UCB-MSCs was successful, indicating that they could be a possible future cell source for bone tissue engineering. The results of this study indicated that the pDBM scaffold could support UCB-MSC cell growth. Additionally, UCB-MSC osteogenic differentiation proved to be successful on this scaffold, as confirmed by SEM, RT-PCR, and the alkaline phosphatase and osteocalcin assays performed. In vivo studies indicated that these differentiated scaffolds were very successful in bone regeneration as shown by the Micro-CT scans, BMD readings, and histological examinations.
Overall, this paper did a fair job in covering its bases and using multiple ways to confirm its results, or explaining any limitations or unexpected finding in the study, such as how the scaffold and bone densities were hard to distinguish from each other in micro-CT scans, or how non-induced cells still had alkaline phosphatase activity. In both cases, because multiple tests were done, such setbacks could be countered by plausible explanations. However, there were a few points that I, as a reader, was concerned about. First, I felt that the cell proliferation should be measured not only by a DNA fluorescence assay, but supplanted with actual cell counting/live dead assay. The DNA measurements could be affected easily by any contamination or the presence of DNA from dead cells. Both of these limitations could be picked up by the cell counting methods. Additionally, I think that RT-PCR and such assays should have also been done on the various differentiated UCB-MSC’s which were first tested on just the culture plate. That way, the effect of the scaffold on levels of gene expression of the osteogenically-induced cells could be compared. Lastly, I was somewhat skeptical by the lack of any immunological response that was reported by the study. During the characterization of the UCM-MSCs, mouse antibodies easily attached to the cells and were used in testing. I found it difficult to believe that a mouse antibody would stick so readily to the cell culture, but a rat would not produce any significant immunological response.

6 comments:
those induced vs. non-induced staining picture are so great!
one question, if the traebecular bone has already been cleaned of cells and mineralization, is the remaining scaffold just composed of protein?
Yuan Fang: I think the pDBM is not fully demineralized, so it is basically partially mineralized collagen with some other proteins.
There are other types of scaffolding for bone besides pDBM, such as ceramics, bioglass, or other materials (such as chitosan-alginate). Each have their own mechanical properties but they are all generally osteogenic and osteoconductive.
One question that I have is that if use of pDBM becomes a medical treatment, where will the pDBM's be collected from? As you said, pDBM's should produce an immune response if they are allografts or xenografts. It wouldn't make sense to harvest bone from the patient and use it for pDBMs. The same question could be asked for obtaining UCB-MSCs.
Another question I have is: what was in the osteogenic medium that induced UCB-MSCs to differentiate into osteoblasts? Is it bone morphogenic proteins or something else?
Do you have any idea what the critical size of a bone defect is where a treatment such as this would be necessary? Also, if this were to become a viable treatment, how would the umbilical cord blood be stored from birth until it is needed?
Do you know how they limited the growth of the new bone to only the fractured area? I would think that whatever factors they used to induce differentiation in the cells would act on areas outside of the desired area as well, through diffusion or other means. This could potential lead to the generation of spurs and irregularities in the newly formed bone, generating a whole slew of problems like inflammation, problems articulating in joint regions, or general discomfort of the test subject (although this could be difficult to gauge in rats)
Eugene: I believe it would be difficult to find critical bone defect sizes for humans due to the impossibility of experimentation. You can easily find critical defect sizes for some other animals, like mice, rabbits, and dogs.
I've heard people can pay for their babies' umbilical cord blood to be frozen for future use.
Yuan:
Bo's right. The scaffold is not fully demineralized. I think they demineralize it is to simply create spaces for the cells to grow. However, the bone minerals are still present in the scaffold
Bo:
As far as the paper is concerned, the pDBM is collected from porcine trabecular bone. You're right that they dont want to harves bone from the patient and use it for a pDBM. In fact, that process is actually similar to current treatments, where bone is taken from the patient and is ground to make bone graft, which is then used in bone regeneration.
As for the osteogenic medium, it is a combination of 10^-8 M dexamethazone, 10mM beta-phospherglycerol, and 0.2M ascorbic acid.
Bo and Eugene:
As for obtaining and storing UCB-MSC's, Mimi is right. The cells are obtained and frozen from the umbilical cord blood of a fetus, provided the mother approves.
Eugene: I am not sure, but I think in humans that the critical size is approximately 7mm.
pattington: while the paper does not address this specific problem directly, I don't think it would be as big of a problem, since they arent using bone morphologic proteins to induce osteogenesis. The bmp's are more likely to induce bone growth in surrounding tissue, but the media the stem cells are exposed to, which may upregulate BMP transcription within the cell scaffold only
Post a Comment