Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart
Harald C Ott, Thomas S Matthiesen, Saik-Kia Goh, Lauren D Black, Stefan M Kren, Theoden I Netoff & Doris A Taylor
Summary:
The Taylor group at the University of Minnesota published this paper in Nature Medicine in early 2008 describing their newly developed method for the growth of a bioartificial heart, using an existing heart as a starting point. This is done in vitro by first decellularizing the original heart, leaving behind an empty "scaffold" of extracellular matrix, or ECM. This shell of ECM is then seeded with fresh neonatal cardiac cells, and incubated in either a perfused bioreactor or a static 2-D culture. Upon characterization of the resulting heart tissue samples and whole organs, it was found that while full physiologic contractile capacity was not obtained, the new hearts were indeed contractile in response to electrical stimulus, demonstrating the potential viability of this technique for the generation of artificial hearts.
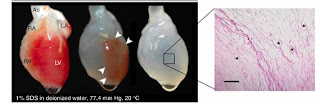
In perfusing the whole-organ heart, three different detergent solutions were used and the relative effectiveness of each was compared. Between solutions of sodium dodecyl sulfate (SDS), polyethylene glycol (PEG), and Triton X-100, the SDS detergent was the most effective at decellularizing the organs.
These decellularized hearts were characterized with a variety of techniques to confirm that they were indeed free of cellular materials. Basic H&E stains were done to visualize heart tissues after treatment with detergent, and qualitatively examined for cells. DAPI stains for nuclei were done to verify the absence of nuclei following detergent treatment, as well as similar stains for cellular actin and myosin to confirm full decellularization. The mechanical properties of the decellularized constructs was also measured; the tangential of the decellularized tissues was higher than that of the cadaveric rat tissues, and similar to that of raw elastin and collagen matrix, as expected.
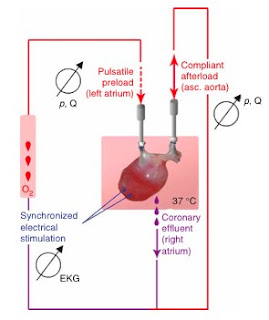
With the sectioned samples, contraction could be elicited with electrical stimuli within the 9th day of growth, up to a frequency of 4 Hz. The perfused tissues, however, were capable of generating contractile forces some 300-800% that of the non-perfused tissues. Furthermore, the size of cardiac muscles generated in the non-perfused samples was limited to ~50 microns in diameter, while the perfused tissues were able to generate viable myofibers up to 1.1 mm thick.
Characterization of the whole heart focused on examining the ability of the recellularized hearts to generate physiologic contractile forces in the ventricles against an afterload. The hearts grown in perfused conditions were capable of generating 2.4 mmHg of contractile force in response to electrical stimulation of up to 4 Hz, with diminishing force as the frequency was increased.
Significance:
Previous studies had already demonstrated to viability of the decellularization technique in generating a matrix scaffold onto which cells can be seeded, but this group is the first to have gone beyond the tissue level and applied the technique to an entire organ. At the time of publication, the "viability" of the heart constructs generated was still limited - the 2.4 mmHg contractile force achieved is only ~2% of the capacity of a typical adult rat heart. However, the authors were optimistic in their conclusions, highlighting the fact that the heart constructs even had fuctional, competant valves. The fact that the cells in the regenerated organ were capable of depolarizing and repolarizing in response to electrical stimuli, generating measureable mechanichal contractions, demonstrates the potential for this technique as a method for growing replacement human organs for transplant.
- David Li

2 comments:
This is great and I hope that we can grow more functional organs and different types in the future. But im confused on the decellularizing process. For this experiment it makes sense because they are seeing if this method will work. But what about for the future regarding using these organs for humans? Is the plan to take a heart from a cadaver, decellularize it, then regrow it with cells from the patient who needs the heart to prevent an immune response? Also, why did they use neonatal cardiac cells?
This sounds very cool! Like Justin, I'm a bit confused on how this works. What kind of diseases would require this perfusion process to be done? It seems like you need the ECM to carry out this process. Why not just do a heart transplant? Is it possible to take a human heart from a living human with some heart diseases, decellularize it, recellularize it, and then put it back to the same person? You mention that after this process, the heart even has a functional valve. How did they test this? Do the valves have the same hemodynamics?
Post a Comment