Tissue Engineering of Complex Tooth Structures on Biodegradable Polymer Scaffolds
C.S. Young1, S. Terada2, J.P. Vacanti2, M. Honda3, J.D. Bartlett1*, and P.C. Yelick1* 1Department of Cytokine Biology and Harvard-Forsyth Department of Oral Biology, The Forsyth Institute, Boston, MA 02115, USA; 2Department of Surgery, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; and 3Department of Oral and Maxillofacial Surgery, Nagoya University School of Medicine, Nagoya, Japan; *corresponding authors, pyelick@forsyth.org, jbartlett@forsyth.org | J Dent Res 81(10):695-700, 2002 (http://jdr.iadrjournals.org/cgi/content/abstract/81/10/695)
Wh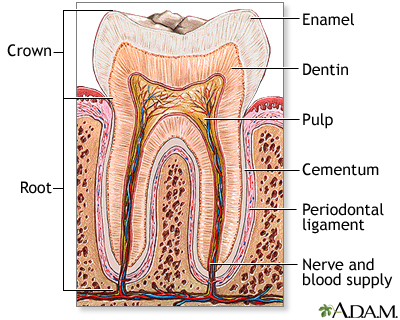 y Study Tooth Tissue Engineering? (other than the fact that teeth are simply awesome!)
y Study Tooth Tissue Engineering? (other than the fact that teeth are simply awesome!)
Trauma, dental caries, and a variety of genetic disorder such as amelogenesis imperfecta can result in a tooth loss, for which there is no regenerative biological substitute currently. This particular area of research has been flourishing since the Forsyth Institute (Boston, MA) presented a possibility of generating bioengineered dental tissues after they found that bioengineered dental tissues were constructed using mixed population of cultured post-natal tooth bud cells (British Dental Journal (2006); 201, 73. doi: 10.1038/sj.bdj.4813885). In order to investigate a tissue engineering approach to construct a biologically suitable substitute for a tooth, this paper I chose (coincidentally it’s from the Forsyth Institute) examines a tissue engineering approach that was used to bioengineer small intestines successfully (Choi and Vacanti, 1997).
What does this study aim to discover in order to demonstrate a regenerative tooth crown?
The signaling between dental mesenchyme that differentiates into pulp and dentin tissues and epithelial tissue of the enamel that produces the dental enamel plays a pivotal role in the overall development of teeth: allowing each tooth to possess different characteristics such as its specific size, shape and function within different positions in the jaw. Thus, this study aims to find the presence of epithelial and mesenchymal dental stem cells in porcine third molar tissues that allow for a successful construction of tooth crowns from dissociated tooth tissues.
*Summary*
Biodegradable polymer scaffolds (a human tooth-shaped polymer scaffolds made with PGA/PLLA and PLGA) were used as substrates to seed dissociated cells from tooth tissues. Third molar tooth buds were obtained from 6 months old pig jaws; pulp organ tissues and enamel tissues were minced after. This cell/polymer constructs were implanted into a suitable host (rat) to allow for the growth of higher-ordered structures. Histological, immunohistochemical, and Laser-capture Microdissection and Reverse-transcription/Polymerase Chain-reaction (LCM, RT-PCR) analyses were on samples from 20, 25, and 30-weeks of implants.
Overall, molecular evidences found from the noted analytical methods indicated a successful bioengineering of complex tooth crowns that closely resemble naturally developing teeth. After 20-weeks of implanting, mineralized dentin, pre-dentin, and pulp tissue resembling vascularized mesenchyme were present. Amelogenin, one of the most prominent extracellular matrix proteins in developing enamel was immunodetected in the enamel matrix of 25-week implant as well as the presence of ameloblast-like cells adjacent to the enamel. 30-week implant tissues showed a thick layer of enamel surrounding a layer of dentin. Amelogenin was detected at the secretory ends of ameloblast-like cells, confirming the occurrence of amelogenesis in the developing enamel. LCM and RT-PCR using nested primers for odontoblast-specific DSPP mRNA confirmed the identity of putative odontoblast-like cells present in bioengineered tooth tissues. Given these morphological, histological, and immunochemical evidences from 20-, 25-, and 30-week implant tissues, this study overall demonstrated a successful construction of bioengineered teeth containing the similar organization and molecular markers of natural teeth such as dentin, pulp, and amelogenin proteins.
Why Did I Choose this Paper?
The approach (seeding dissociated tooth tissues onto biodegradable scaffolds) this paper took signifies a possibility of generating teeth of preprogrammed size and shape, giving engineers more control over the overall function and morphology of bioengineered tooth. The bioengineered tissues not only provided morphologically evidences of natural teeth, LCM and RT-PCR further confirmed the on-going process of amelogenesis. Since my research interest lies within dental science, specifically in understanding amelogensis by closely mimicking the enamel formation in vitro, I chose this paper to study possible approaches engineers can take in regenerating teeth that closely resemble the natural teeth. If we can understand how biological system is able to design organized structures (ie. enamel) in tissues at the molecular level, not only people with genetically impaired teeth could recover their full dentition but we may even be able to use artificial human enamel to build a dental restoration. For instance, if the molecular mechanism of amelogensis was known, engineers can further manipulate the developmental process of bioengineered teeth to produce fully-developed enamel.
*Here are some interesting papers you can read at your leisure:
- Micelle Structure of Amelogenin in Porcine Secretory Enamel: J Dent Res 86(8):758-763, 2007 (http://jdr.iadrjournals.org/cgi/content/abstract/86/8/758)
- Bioengineered Teeth from Cultured Rat Tooth Bud Cells: J Dent Res 83(7): 523-528, 2004 (http://jdr.iadrjournals.org/cgi/content/full/83/7/523)
- New observations of the hierarchical structure of human enamel, from nanoscale to microscale: Journal of Tissue Engineering and Regenerative Medicine V1 I3: 185-191 (http://www3.interscience.wiley.com/cgi-bin/abstract/114219401/ABSTRACT)

5 comments:
What are a few of the challenges specific to engineering teeth (as opposed to other organs)?
One challenge is manipulating growth rate of the artificial teeth in order to efficiently and rapidly grow hydroxy apatite (HAP) crystals on DEJ (Dentino-Enamel Junction) which will fully mature into enamel (outermost layer in our teeth; hardest tissue in our body as well) with all functional properties our teeth possess. In vivo, enamel fully develops at a very slow rate (enamel mineralization is completed 1.5 months to 10 months after birth). If we take biomimetic approach to engineer artificial teeth, no patient would want to wait 10 months to fully develop functional enamel in their oral cavity. Furthermore, although enamel is 96%wt mineralized, its growth is known to be guided by amelogenins, organic molecules. Thus, protein-protein interactions, organic-inorganic interactions, and inorganic-inorganic interactions need to be understood at the molecular level to successfully engineer teeth.
On a side note, the high content of enamel is susceptible to deconstruction (ie. through dental caries) as well. Therefore, it'd be definitely interesting and challenging to engineer artificial teeth (by possibly mimicking natural enamel, yet enhancing its functionality somehow) that may not as susceptible to soft drinks, sugars, and all the sweet things.
If I can think of more in the future, I will surely post :) Thanks for the question, Terry!
Cool stuff! But I don't really understand the controls that they did. They talked about controls during the staining, but they didn't really describe the control conditions. Thanks!
"demonstrated a successful construction of bioengineered teeth containing the similar organization and molecular markers of natural teeth such as dentin, pulp, and amelogenin proteins." Does that mean they actually grew a tooth in culture?? I can't really see how in vivo conditions would be similar enough to the culture conditions for the same growth to occur. Is this meant to be grown in vitro then transplanted? If so, is real teeth really that much better than synthetic teeth materials?
Jen, this is a really interesting article! I think I might be confused about the same thing as the poster above me. Does this mean that they are grown in vivo and will be transplanted eventually to a human or do you mean that they want it to be implanted in human teeth and have it regenerate there?
Also, how do they control size and shape of the tooth? i.e. will there be limitations on which kinds of teeth (molars, incisors, etc.) that can be made?
Post a Comment