Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts
Citation
Hashi, Craig K., Yiqian Zhu, Guo-Yuan Yang, William L. Young, Benjamin S. Hsiao, Karin Wang, Benjamin Chu, and Song Li. "Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts." Applied Biological Sciences. Pubmed.
Significance
Cardiovascular disease is the leading cause of death in the
SUMMARY
Fabrication
Poly(L-lactic acid) (PLLA), an FDA approved biocompatible, biodegradable polymer, was used to electrospin 50 μm thick, highly porous scaffolds with nanofibers »0.5-1μm in diameter and circumferentially aligned. These fibers had a Young’s modulus of 12 MPa, similar to that of the native vessel. MSCs were seeded onto these nanofibrous membranes and rolled around a .7-mm mandrel. This procedure yielded a tubular shaped membrane with MSCs embedded within the walls of the graft. A live/dead assay was performed one day after incubation to assess cellular response to physical handling: almost all cells were found to be calcein-positive (live) and ethidium bromide-negative (dead). The vascular graft was implanted into the common carotid artery (CCA) of a rat 3 days after incubation. A live/dead assay was performed on a graft adjacent to an MSC graft to assess cellular response to suturing: almost all cells were found to be calcein-positive (live) once again.
The grafts were implanted into the rats for up to 60 days, and the remodeling of vascular grafts in vivo was later assessed. Native blood vessels have three distinct layers: endothelial cell (EC) monolayer, a medial smooth muscle cell (SMC) layer embedded in a three-dimensional ECM (mainly collagen and elastin), and an adventitial layer of connective tissue. In contrast to nanofibrous grafts alone, MSC seeded grafts showed minimal intimal thickening or hyperplasia (Fig. 1 a vs b). Cross-sections of the vascular grafts were stained for CD31 and myosin heavy chain (MHC) to locate EC and SMC, respectively, in order to determine cellular components and organization (Fig. 1 c, d, e, and f). ECM remodeling was also analyzed by staining cross-sections of MSC-seeded and acellular grafts for collagen and elastin. An elastic lamina layer adjacent to the lumen was only observed in the MSC-seeded grafts (Fig. 1 h); diffuse elastin staining was observed in the acellular grafts (Fig. 1 g).

Figure 1 (to right): a) accelular versus b) MSC-seeded graft c) acellular versus d) MSC-seeded graft stained for CD31 (ECs) e) acellular versus f) MSC-seeded graft stained for MHC (SMCs) g) acellular versus h) MSC-seeded grafts stained for elastin (black) and collagen (pink)
Antitrhombogenic properties of MSCs in Vivo

Figure 2: SEM images of platelet adhesion to the luminal surface of a) acellular and b) cellular grafts 2 hours after implantation. H&E staining (c and d) and birefringence images (e and f) of acellular versus MSC-seeded grafts 3 hours after implantation g) significant intimal hyperplasia for acellular grafts
Because thrombosis is the main cause of failure in small-diameter vascular grafts, platelet adhesion to MSC and acellular grafts was analyzed. SEM images revealed significant amounts of platelet adhesion to the acellular graft two hours after implantation, whereas minimal platelet adhesion was observed to the MSC-seeded graft (Fig. 2 a and b). Cross-sectional staining also showed thrombus formation on the luminal surface of the acellular graft but not the MSC-seed graft (Fig. 2 c and d). Furthermore, the antithrombogenic property of MSCs was compared to that of ECs and SMCs. In vitro experiments were performed to determine platelet adhesions on surfaces coated with gelatin (positive control), ECs, SMCs, and MSCs. CD41—a platelet-specific marker—was used to identify adherent platelets. Significantly lower platelet adhesion was observed on MSCs and ECs than on SMCs (Fig 3 g).
Both MSC-coated and acellular grafts showed efficient recruitment of vascular cells and organized layers of ECs and SMCs. Because few MSCs were found in the grafts after 2 months, the specific role of MSC in cell recruitment is unclear. The acellular grafts did, however, show intimal hyperplasia, whereas the MSC coated grafts did not. In designing the synthetic scaffold, an appropriate nanofiber density and porosity is required for efficient cell infiltration and the maintenance of mechanical strength. Furthermore, the antiplatelet adhesion property of MSCs was found to depend on the cell-surface expression of heparin sulfate proteoglycans—treatment with heparinase II significantly increased platelet adhesion on MSCs and ECs (Fig 3 h). This finding provides a rational basis for coating the nanofiber surfaces with antiplatelet adhesion/aggregation molecules. Besides the antithrombogenic properties of MSC, MSC’s role in ECM remodeling and other early phase postimplantation processes is unclear. The use of bone marrow stem cells to construct tissue-engineered vascular grafts (TEVG) may have significant clinical implications. The patient’s own bone marrow or a cell bank of MSCs may be used to fabricate allogenic grafts. Alternatively, the surface of acellular grafts may be modified with certain molecules such as heparin to be made antithrombogenic. 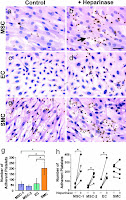
Figure 3: Platelet adhesion stained with H&E (dark brown) to MSCs, ECs, and SMCs without (a,c,e) and with the addition (b,d,f) of heparinase. g) number of adherent platelets on MSCs, ECs, and SMCs after 30-min adhesion (n=4, p<.05) h) the effects of heparinase II treatment on platelet adhesion (n=4, p<.05)

1 comment:
Since Poly(L-lactic acid) (PLA) is biodegradable, does it gradually degrade after implantation?
You said “minimal platelet adhesion was observed to the MSC-seeded graft.” If the MSC-seeded graft was antithrombogenic, why did the nanofiber surface still need to be coated with antiplatelet adhesion/aggregation molecules? Was the acellular graft treated with antiplatelet adhesion/aggregation molecules studied? What is the significance of MSC-seeded graft if the same goal can be achieved simply by coating it with antithrombogenic treatment?
Post a Comment