Apatite content of collagen materials dose-dependently increases pre-osteoblastic cell deposition of a cement line-like matrix.
Reference
Perrier A, Dumas V, Linossier MT, Fournier C, Jurdic P, Rattner A, Vico L, Guignandon A. Apatite content of collagen materials dose-dependently increases pre-osteoblastic cell deposition of a cement line-like matrix. Bone 2010.
Summary
The authors showed the increase in osteoblast activity during bone remodeling process is attributed to pre-osteoblastic secretion of VEGF-A and expression of RGD-containing proteins, osteopontin and fibronectin. Bone breakdown exposes varying degree of mineralization (dom) within a collagen I matrix; pre-osteoblast cells encounter such structure and release the above factors to promote cell viability and adhere to the collagen I-hydroxyapatite composite. The researchers evaluated this by observing cell morphology, mRNA production, adhesion rate, and cell migration. With increasing dom, the cells took on a more unique morphology, produced more mRNA for VEGF-A and RGD-containing proteins, displayed higher adherence rate, and migrated larger distances.
Results
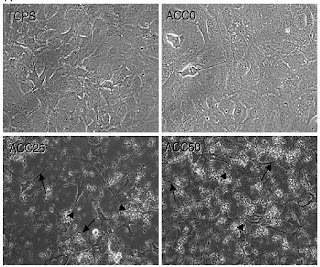
The Apatite Collagen Complex (ACC) was made in 3 groups: 0%, 25%, and 50% (as the human bone is max 42%) apatite degree of mineralization. The ACC was first characterized as a substrate under light microscopy to quantify (via ImageJ) the amount of collagen and degree of mineralization was easily distinguishable. The ACC substrates were then tested for biocompatibility by seeding MC3T3-E1 cells; the light microscopy images are shown below. As the dom increases, the cells take on a more elongated, ciliated, stretched morphology showing adhesion and migration.
mRNA of VEGF-A was quantified using ELISA which as the figure below indicates, the 25% and 50% show marked, dose-dependent increases from 0% to 25% to 50% both in soluble VEGF-A and matrix-bound. The RGD-containing proteins OPN & FN were also shown to increase in mRNA content by RT-PCR; OPN increased 50-fold for 25% & 40-fold for 50% whereas FN increased by 7-fold for 25% and 8.5-fold for 50%. B3 integrin, the RGD-containing protein cell surface receptors, were also upregulated by 3-fold to 4-fold 25%-50%, respectively.
From protein expression, the authors moved on to the manifestation of increased expression by mechanically testing cell adhesion. A vertical sinusoidal vibration was applied to the cells; collagen I indeed did facilitate better adhesion and as the dom increased, the adhesion also increased dramatically: 4.59+0.82% cells resisted (TCPS), 29.00+5.77% (ACC0), 56.17+11.84% (ACC25) and 75.14+6.44 (ACC50). Finally, cell migration was observed using time-lapse microscopy in which the cells’ migration was recorded to move greater distances with increasing dom; it was also found that cells in unmineralized substrates (TCPS & ACC0) had low speed profile while those in 25% and 50% had alternating low and high speed profiles.
Shortfalls
Overall, this paper presents relatively minute shortfalls.
Although the paper used several methods to prove the changing profile of VEGF and OPN/FN, the most basic information of experimental setup was omitted: the authors did not provide a total cell count in the 4 categories they were comparing. Hence, there is no way to tell if the results are statistically significant except relative to each other.
The ACC substrate system was imported from those used in previous osteoclast experiments; osteoclasts, though derived from the same precursor, have ruffled border morphology to aid in adhesion and bone breakdown. The ACC was thus prepared in a way to aid adhesion which can contribute to the increasing cell adhesion and morphological changes to osteoblast with increasing dom, thus presenting a confounding factor.
A type of protein quantification for the RGD-containing proteins OPN, FN, and B3 integrin would have complemented the mRNA results as they had done for VEGF-A. Finally, the choice of 32hr post seeding to conduct experiments seem arbitrary given a doubling time of 27 hours for MC3T3-E1 cells; an scientific rationale would have strengthen the results.


2 comments:
It seems like there was a good variety of characterization methods carried out. Did the paper mention how the ACCs were fabricated, and did they discuss how their findings might be employed in designing clinical treatments or devices?
I'll do this Twitter style for efficiency.
@Janna & Scott
ACCs were created based on a method from a cited paper (Shibutani T et al J Biomed Mater Res 2000;50(2):153–9.)
Basically,cell cultured-treated dishes are coated with collagen I and incubated in 2 successful washes to precipitate calcium:
1) TBS containing alkaline phosphatase and egg yolk phosvitin
2) calcium B-glycerophosphate solution
Cross-linking agent dimethyl suberimidate hydrochloride was applied in between the 2 washes.
The incubation time and amount of washes were modulated to vary the degree of mineralization (d.o.m.).
@Janna: Used TIRAvib vibration generator which looks like this (http://www.bksv.com/doc/bp1910.pdf). You place the slides in the central tunnel and it can produce precise vibrations on any frequency you choose. Low and high speed settings were used for ACC25 & ACC50 to test for migration. It's not important for ACC0 as there is no deposition.
@Scott: No they did not specifically mention how this could translate to treatment modalities or medical devices, but you can infer based on their findings that this allows for better strategies to treat osteoporosis for instance.
Thanks for the insightful questions Janna and Scott. Best of luck on finals.
Post a Comment