3D environment on human mesenchymal stem cells differentiation for bone tissue engineering
3D environment on human mesenchymal stem cells stem cells differentiation for bone tissue engineering
T.Cordonnier, P. Layrolle, Julien Failard, Alain Langonne, L Sensebe, P Rosset, J Sonier
Journal of materials science. Materials in medicine
Received: 30 June 2009/ Accepted 13 October 2009
Springer Science + Business Media, LLC 2009
Due to the increasing demand for bone graft substitutes, Cordonnier et al. sought to study the ability of human mesenchymal stem cells in forming a 3D environment when seeded to synthetic bone substitute, bisphasic calcium phosphate, particles, and the potential differentiation of the hMSCs in such environment. After experimentation, the authors conclude that BCP particles unaccompanied are capable of inducing hMSC differentiation in vitro.
To test their suspicions, they seeded cells on a culture treated polystyrene (2D, control) and on the BCP particle cultures (3D, experimental). Furthermore, they added two different types of media: proliferative media, and osteogenic media, to each culture. Cell proliferation was measured at day 1, 3, 7, and 14 by fluorescence after 30 min incubation of an Alamar blue/PBS solution. An alkaline phosphatase, an important enzyme in bone mineralization, staining assay was performed to test differentiation of the hMSCs to osteoblasts. Real time quantitative RT-PCR was also performed to measure the gene expression throughout the two weeks of experimentation. All experiments were triplicated.
After the first day and some manual homogenization, the authors report seeing a thin monolayer of BCP particle aggregation on the BCP seeded cultures. A scanning electron microscope image was taken of the 3D structures in the cultures after 14 day, revealing a intricate ECM formation that covered the BCP particles; this structure remained throughout the entire experiment. The proliferation assay data shows an increase in hMSC cells up until the 4th day for the BCP cultures in both and osteogenic and proliferative medium, while interestingly the cells proliferate until the 14th day on the PSTC plastic. The authors suggest that this data implies mass osteoblastic differentiation of the hMSCs due to the 3D environment. Moreover, their ALP assay results seemingly affirms this position; the data shows that the cells seeded on BCP particles without osteogenic factors show higher expression of ALP than the 2D plastic cultures. But because ALP expression does not undoubtedly confirm differentiation, so they turn to their RT-PCR data. According to the RT-PCR, in proliferative media, there was higher expression of BMP-2 and BSP on the BCP particles compared to the plastic. And as expected the osteogenic medium cultures showed higher expression of ALP and BSP than the proliferative medium, except for BMP-2. Interestingly, the plastic culture with osteogenic medium data looked very similar to the BCP particle with proliferative medium data.
So the authors finally conclude that the hMSCs can attach to BCP, proliferate, and form 3D structure that ultimately leads to osteoblastic differentiation.
My main complaints are that the authors fail to explain the lower expression of BMP-2 in the osteogenic medium, and they completely neglect the increased proliferation of hMSCs on the BCP particles after day 14 (Figure 2). More nit-picky points are that they choose a very small sample of cells from 3 healthy patients and they only reproduced their experiments 3 times. A suggestion might be to do a quantitative protein assay to look at the existence of protein and their concentration because mRNA does not always mean protein.
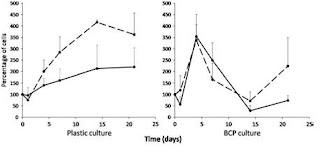 Figure 2 hMSC proliferation on different substrates. A fraction of cells was culured on PSTC plastic and another was cultured on BCP ceramic particles in proliferative (dotted lines) or osteogenic medium (full lines) during 21 days.
Figure 2 hMSC proliferation on different substrates. A fraction of cells was culured on PSTC plastic and another was cultured on BCP ceramic particles in proliferative (dotted lines) or osteogenic medium (full lines) during 21 days.

Figure 3 SEM of 3D environments after 14 day

7 comments:
I'm curious to know what MSCs cell density was used for the Bisphasic calcium phospate (BCP) particle cultures.
Also the geometry of the BCP particles are also important. Was it a porous microenvironment or was it non-porous to enhance MSC differentiation?
According to the article, they chose a cell density of 2*10^6 cells per 160 mg BCP particles. But they do not specify the amount of BCP particles,so I am not sure how many cells that is exactly.
I think it is implied that the BCP particles form a base for the seeds to grow on top of, but the cells themselves create a 3D environmental composed of ECM/BCP particles afterwards. In the discussion, the microenvironments are said to be porous.
Do the authors talk in detail about what the SEM images suggest about the proliferation or differentiation of cells in BCP seeded cultures? Were they trying to show from the images that the ECM produced by these cells is similar in structure to that produced by osteoblasts?
If they're investigating the potential of these cells for bone graft substitutes, then it would be very interesting to go even further and compare the composition and properties of the ECM produced by the cells in these various growth situations. And then they would want to compare those results to the ECM created by actual osteoblasts. If they could manipulate the hMSC's to create ECM similar to real human osteoblasts, then that would be the ultimate goal, right?
One change that I might make to the experimental design would be to create a better control than TCPS. If the BCP surface was designed to become a 3D culture through cell ingrowth, then a similar control should be created. Perhaps a TCPS surface with polystyrene beads in the same size scale and density as the BCP molecules? Either way, it seems like the way the authors do it neglect to look at effects of different starting substrates.
To address Harry's comment, the authors do not explicitly state anything about the SEM images and ECM from osteoblasts. The presence of ECM does suggest proliferation though.
Ultimately they want to be able to make bone grafts from the porous 3D scaffolds and differentiated-hMSC oseteoblasts.
The premise of the paper is to create a scaffold for HMSCs on BCP particles in order to mimick the conditions of the bone in order to create a better scaffold. However, to make a realistic scaffold, it seems that they are missing a couple experiments.
Do they do any tests to actually see the mechanical properties of their scaffold? Furthermore, is the scaffold biodegradable such that normal bone can replace the scaffold over time?
Those are great questions. The short answer is that they don't. To ensure that these scaffolds would be a feasible treatment, they would have to examine their mechanics maybe by AFM. So they should do further anaylze if this material can be absorbed possibly by seeded osteoclasts.
Post a Comment